
Inorganic Chemistry Department, South Parks Road, University of Oxford,OX1 3QR, UK
e-mail: nan.wang@chemistry.oxford.ac.uk
 |
Nan Wang and Jason J. Davies
Inorganic Chemistry Department, South Parks Road, University of Oxford,OX1 3QR, UK e-mail: nan.wang@chemistry.oxford.ac.uk |
 |
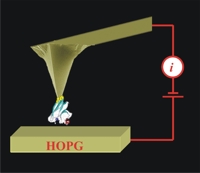
There is no doubt that the protein would serve as molecular electronic material for the next generation of nanoelectronic devices. The protein stability including dielectric stability and mechanical stability is a crucial issue in its application in molecular electronics. It predominates the reliability and performance of electronic devices. In this work, a new method for characterising the dielectric and compressional mechanical strengths has been developed with C-AFM using a tip modification strategy. The metalloprotein, azurin was chosen to study due to the great significance in respiratory and photosynthetic electron transport chains.
Under a force lower than 2 nN, dielectric breakdown has been observed at a bias higher than a critical value at either positive or negative polarization. The dielectric strength of protein is between 1.0 ~ 1.4 GV/m, indicating that the protein can be a good molecular electronic material with sufficiently high dielectric strength. When the force is between 2nN-5 nN, the I-V curves of Cu centre azurin show NDR on higher bias while the redox inactive Zn substituted one is absent of resonance. When reliable electrical contact between electrodes and protein is achieved under a force greater than 5 nN, well-behaved current-voltage characters are revealed, and dependent on the force load. The mechanical strength of protein has also been elucidated on basis of the fact that the extremely high force ranging from 60 to 100 nN leads to collapse of the protein molecular structure.