Nanoscience Group, Materials Chemistry Division, National Chemical Laboratory, Pune – 411 008, India
e-mail: sastry@ems.ncl.res.in
| Atul Bharde and Murali Sastry
Nanoscience Group, Materials Chemistry Division, National Chemical Laboratory, Pune – 411 008, India e-mail: sastry@ems.ncl.res.in |
 |
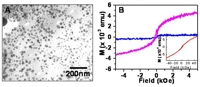
Formation of inorganic nanocrystals has been considered to occur mainly via chemical processes without much participation of biological activity. Biological synthesis of inorganic materials is carried out under ambient conditions of temperature, pH and pressure. Some well known examples include synthesis of calcium carbonate and calcium phosphate in S-layer cyanobacteria [1], gold in fungi [2], sponge and diatomic silica [3] and magnetite or greigite in magnetotactic bacteria [4,5]. There is much current interest in developing nanoparticulate magnetite due to its potential for application in multi-terabit magnetic storage devices, ferrofluids, contrast enhancers in magnetic resonance imaging and as biological markers. Current chemical synthetic methods for magnetite are energy intensive, employ toxic chemicals and often yield particles in organic solutions there by precluding biomedical applications. Biosynthesis of magnetite and greigite is exclusively within the realm of magnetotactic and iron reducing bacteria which require anaerobic conditions [4,5]. We discuss here formation of magnetite and greigite by the reaction of an aqueous solution of iron salts with the Gram-positive bacterium Actinobacterium spp. under aerobic conditions. Magnetite was formed in the presence of a 2:1 mM mixture of K3Fe(CN)6 and K4Fe(CN)6 while greigite was produced in presence of ferric citrate and ferrous sulfate in 2:1 mM ratio respectively. The nanoparticle products were characterized by a host of spectroscopic and microscopic techniques.