
Institute of Biochemistry, Goethe-University of Frankfurt, Marie-Curie-St. 9, D-60439 Frankfurt/Main, Germany
e-mail: j.piehler@em.uni-frankfurt.de
 |
Suman Lata, Annett Reichel, and Jacob Piehler
Institute of Biochemistry, Goethe-University of Frankfurt, Marie-Curie-St. 9, D-60439 Frankfurt/Main, Germany e-mail: j.piehler@em.uni-frankfurt.de |
 |
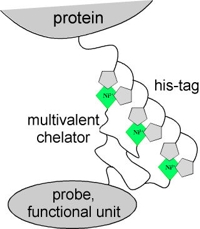
Organizing and manipulating proteins on the molecular level requires adaptors for attaching spectroscopic probes and other functional elements in a site-specific and structurally defined manner. We have engineered chemical recognition units, which bind oligohistidine tags with high affinity and stability. Several supramolecular entities containing 2-4 NTA moieties were synthesized, additionally presenting an orthogonal group for coupling functional units or spectroscopic probes. These multivalent chelator heads (MCH) – termed bis-, tris- and tetrakis-NTA – were characterized with respect to their interaction with histidine-tagged targets. Stable and stoichiometric labeling of histidine-tagged proteins was achieved by tris- and tetrakis-NTA. Kinetic measurements revealed an increase in stability by 4 orders of magnitude compared to mono-NTA, and sub-nanomolar affinity was reached for tris-NTA. Yet, very fast detachment of these adaptors by competing ligands was possible. These novel biochemical tweezers were used for functional immobilization of proteins on fluid and non-fluid supports using tailored histidine-tags for differential recognition. Furthermore, fluorescence probes were coupled to these MCH for non-covalent fluorescence labeling. Highly specific labeling was obtained, enabling for protein interaction and conformation analysis in complex matrices. Furthermore, specific labeling of transmembrane receptors in living cells was achieved. Numerous applications of these biochemical tweezers in nanobiotechnology can be envisaged.