
1M. Lomonosov Moscow State University, Physics Department, Moscow, Russia, and
2Engelhardt Institute of Molecular Biology of the Russian Academy of Sciences, Moscow, Russia
e-mail: nvm@lt.phys.msu.ru
 |
V.N. Nikiforov1, Yu.D. Nechipurenko2, V.I. Salyanov2, and Yu.M. Yevdokimov2
1M. Lomonosov Moscow State University, Physics Department, Moscow, Russia, and
e-mail: nvm@lt.phys.msu.ru |
 |
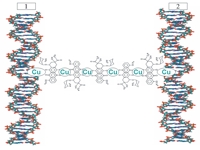
The formation and the properties of DNA-daunomycin-Cu2+-nanoconstruction were studied. It consists of the double-stranded molecules of nucleic acids located at a distance of 30-50 Å in the spatial structure of particles of their cholesteric liquid-crystal dispersion (LCD) and crosslinked by artificial nanobridges. The resulting nanostructures possess the peculiar physicochemical properties.
The temperature dependence of magnetic moment, Pm, in liquid-crystal dispersions was experimentally measured by SQUID magnetometer. Using the temperature dependence of the magnetic moment upon temperature, the magnetic susceptibility (χ) was calculated.
It was shown that the magnetic susceptibility contains the contributions both from paramagnetic and diamagnetic centers. The paramagnetic centers are only ions Cu2+. The basic diamagnetic contribution is expected to be caused by water molecules and benzene rings of Dau. At low temperatures the magnetic susceptibility is positive, whereas at high temperatures this is negative. Hence, the total magnet moment is represented by two parts, i.e. the positive paramagnetic part, caused by Cu2+-ions, and the negative diamagnetic part, caused both the presence of the "rest" water and benzene rings of Dau.
Copper ions are participating in the formation of nanobridges within NaC. In this case each nanobridge, located in each helical turn between the neighboring DNA molecules, contains approximately 6 copper ions.