
Chair of Biophysics, Ruhr-University, D-44780 Bochum, Germany
e-mail: gerwert@bph.rub.de
 |
Klaus Gerwert
Chair of Biophysics, Ruhr-University, D-44780 Bochum, Germany e-mail: gerwert@bph.rub.de |
 |
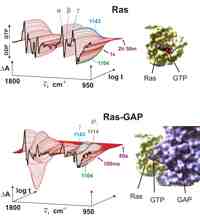
Time-resolved FTIR difference spectroscopy can be used to monitor the reactions within proteins at atomic resolution. The difference technique resolves the active groups from the background absorbance of the entire protein. In combination with site directed mutagenesis or isotopically labeling the IR bands can be clear cut assigned to the protein groups, introduced in [1]. Based on first fast scan studies on bacteriorhodopsin a detailed proton pump model is proposed [1,2]. This is in nice agreement with MD simulations based on the 3-D structural models of bacteriorhodopsin [3]. Based on succeeding step scan FTIR measurements a proton transfer via a protonated H-bonded network of internal water molecules is shown for bacteriorhodsin [4] and might also present in the photosynthetic reaction center [5]. The approach is also successfully applied to the photoactive yellow protein [6].
Recent progress for investigation of processes in non chromophoric proteins is established by developing a micro mixing cell for FTIR studies [7].
Using caged GTP the GTPase mechanism of the protooncogen Ras is investigated [8,9] and its protein protein interaction with GAP [10].