
Bionanotechnology Interdisciplinary Research Consortium, Clarendon Laboratory, Department of Physics, Oxford University, Parks Road, Oxford OX1 3SU, UK
e-mail: wolfgang.fischer@bioch.ox.ac.uk
 |
Wolfgang B. Fischer
Bionanotechnology Interdisciplinary Research Consortium, Clarendon Laboratory, Department of Physics, Oxford University, Parks Road, Oxford OX1 3SU, UK e-mail: wolfgang.fischer@bioch.ox.ac.uk |
 |
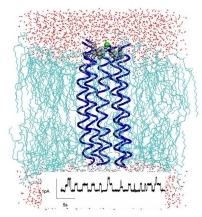
All known viral ion channels to date are approximately five times smaller than the ion channels found in our body. We need to investigate the mechanistic details of these viral ion channels because of their biomedical relevance. For a future perspective, their small size makes them suitable for analysis by protein chemistry and for large scale production and application in Bionanotechnology. Ion channels are atomic scale valves that control macroscopic flows and can be used in sensors, amplifiers, diodes, filters and batteries.
Current investigations focus on Vpu from HIV-1 which is known to form ion channels by self-assembly if reconstituted as full length protein into lipid bilayers. Also peptides corresponding to the transmembrane (TM) part of the proteins exhibit channel activity. These peptides can be derived from solid phase peptide synthesis. From detailed NMR spectroscopic studies it is known that the TM part of the protein is helical. Conductance measurements and computer simulations on the TM peptide, Vpu1-32, are undertaken to correlate channel characteristics such as conductance levels (levels as low as 9 pS have been observed with the most populated level of approximately 20 pS), ion selectivity and interactions with modulators (derivative of amiloride) with atomic details.