
Department of Chemistry and Pharmacy, Ludwig-Maximilians-University, Butenandtst. 5-13 (building F), D-81377 Munich, Germany
 |
Guido Clever and Thomas Carell
Department of Chemistry and Pharmacy, Ludwig-Maximilians-University, Butenandtst. 5-13 (building F), D-81377 Munich, Germany |  |
Base pairing in natural oligonucleotides relies on hydrogen bonding and π-stacking. Applying coordinative interactions between ligand-like nucleobases ("ligandosides") and metal cations is a new way of assembling artificial oligonucleotide duplexes [1]. As these systems are hybrid compounds consisting of a metal complex in a chiral DNA environment, enantioselective catalytic activity may be a promising new feature [2]. Incorporation of numerous metallo-base pairs into oligonucleotides may be useful to develop sequences whose ability to hybridise to a counterstrand can be switched by the presence or oxidation state of specific metals in solution.
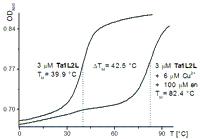
The synthesis of our ligandoside comprises preparation of a glycosyl donor, a suitable protected salicylic aldehyde and an organo-cuprate mediated C-glycosidation as the key step [3]. Subsequently, a phosphoramidite for the use in solid phase DNA synthesis was prepared. Incorporation into various oligonucleotides was followed by deprotection of the salicylic aldehydes, purification and characterisation of the DNA strands. All the synthesised double strands and hairpins with ligandosides facing each other show typical B-DNA CD spectra and distinct melting characteristics, with a melting temperature depression of 9°C compared to the comparable double strands containing an AT base pair instead. Addition of excess ethylenediamine leads to an increase of 5°C, addition of a slight excess of Mn2+ and Cu2+ increases the melting temperature by 28°C and 42°C, respectively.